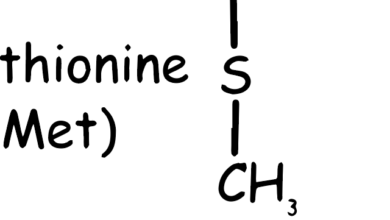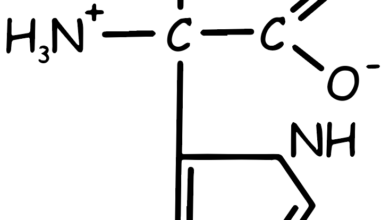Amino Acids: Building Blocks of Life

Amino acids are organic compounds that combine to form proteins, which are crucial for many functions in living organisms. They are characterized by containing both an amino group (-NH₂) and a carboxylic acid group (-COOH), along with a side chain (R group) specific to each amino acid. The key properties and functions of amino acids include:
Amino acids, the building blocks of proteins, form long chains that fold into specific three-dimensional structures, dictating the protein’s function. There are 20 standard proteinogenic amino acids encoded by the human genetic code, alongside non-standard amino acids that are not DNA-coded but remain crucial in biochemistry. Humans must obtain essential amino acids, such as valine, leucine, isoleucine, lysine, phenylalanine, threonine, tryptophan, methionine, and histidine, through diet, as they cannot synthesize all amino acids. These amino acids, vital for protein construction, also contribute to neurotransmitter transport and biosynthesis, immune response, metabolism, and cell signaling. Structurally, an amino acid consists of a central carbon atom bonded to a hydrogen atom, an amino group, a carboxyl group, and a variable R group or side chain, which defines its specific identity and properties. The solubility and reactivity of amino acids depend on their side chains, which can be nonpolar, polar, acidic, or basic. Amino acids are interconnected by peptide bonds, forming peptides or proteins through a bond between the carboxyl group of one amino acid and the amino group of another. The sequence and number of amino acids in a protein are pivotal in determining its shape and function, allowing a vast diversity of proteins in living organisms.
Understanding amino acids is fundamental in fields like biochemistry, molecular biology, and medicine, as they are central to the structure and function of all living cells.
The 20 proteinogenic amino acids are the building blocks of proteins in living organisms. These amino acids are encoded directly by the universal genetic code and are used to synthesize proteins during translation. Each has unique properties that influence the structure and function of proteins. Here’s a brief overview of each:
- Alanine (Ala, A): Nonpolar, aliphatic side chain. It’s small and plays a key role in protein structure and function.
- Arginine (Arg, R): Positively charged at physiological pH, with a complex guanidinium group. It’s important in cell signaling and metabolism.
- Asparagine (Asn, N): Polar and uncharged. Contains an amide group and is involved in protein structure and metabolism.
- Aspartic acid (Asp, D): Negatively charged at physiological pH. It’s acidic and involved in protein structure and enzyme activity.
- Cysteine (Cys, C): Contains a thiol group. It forms disulfide bonds, crucial for protein structure and function.
- Glutamine (Gln, Q): Polar and uncharged. Contains an amide group and is important in nitrogen metabolism.
- Glutamic acid (Glu, E): Negatively charged at physiological pH. It’s acidic and plays a role in protein structure and neurotransmission.
- Glycine (Gly, G): The simplest amino acid, nonpolar, and used in protein construction.
- Histidine (His, H): Positively charged at physiological pH. It contains an imidazole ring and is crucial in enzyme active sites.
- Isoleucine (Ile, I): Nonpolar, aliphatic side chain. It’s important for protein structure and is an essential amino acid.
- Leucine (Leu, L): Nonpolar, aliphatic side chain. It’s essential and plays a key role in protein structure.
- Lysine (Lys, K): Positively charged at physiological pH. It’s essential and involved in protein structure.
- Methionine (Met, M): Contains a sulfur atom. It’s essential and serves as the start codon in protein synthesis.
- Phenylalanine (Phe, F): Aromatic, nonpolar. It’s essential and plays a role in protein structure and function.
- Proline (Pro, P): Contains a unique ring to the N-terminal amine group, impacting protein folding.
- Serine (Ser, S): Polar and uncharged. It’s involved in protein structure and enzyme catalysis.
- Threonine (Thr, T): Polar and uncharged. It’s essential and plays a role in protein structure.
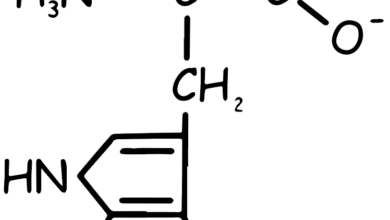
- Tryptophan (Trp, W): Aromatic, contains an indole group. It’s essential and involved in protein structure and function.
- Tyrosine (Tyr, Y): Aromatic, polar. It’s involved in protein structure and enzyme activity.
- Valine (Val, V): Nonpolar, aliphatic side chain. It’s essential and contributes to protein structure.
These amino acids are essential in the synthesis of proteins and play various roles in cellular processes and metabolism. Some are essential, meaning they must be obtained through the diet, while others can be synthesized by the body.
Beyond the 20 standard proteinogenic amino acids, there are several non-standard amino acids that play roles in biological processes. These include:
- Selenocysteine (Sec, U): Often referred to as the 21st amino acid, it’s incorporated into proteins by a unique translation mechanism. Selenocysteine is found in several enzymes, including glutathione peroxidases and deiodinases, and is important for antioxidant protection and thyroid hormone metabolism.
- Pyrrolysine (Pyl, O): Sometimes considered the 22nd amino acid, it’s used by some methanogenic archaea and bacteria in enzymes that are part of methane production. Pyrrolysine is encoded by a specific codon, UAG, which is usually a stop codon.
In addition to these, there are many other non-proteinogenic amino acids found in nature, which are not directly coded by DNA but can have biological roles. For example:
- Ornithine and Citrulline: Intermediate compounds in the urea cycle.
- D-Amino Acids: Found in bacterial cell walls and some peptide antibiotics.
- Beta-Alanine: A component of pantothenic acid (Vitamin B5) and the peptide carnosine.
- Gamma-Aminobutyric Acid (GABA): An important neurotransmitter in the central nervous system.
- N-Methylated Amino Acids: Such as N-methyllysine, found in some proteins post-translationally modified.
These non-standard amino acids are often critical for specific biological functions, including regulation of enzyme activity, signal transduction, and metabolic pathways. They can be derived through post-translational modifications of proteins, biosynthetic pathways, or can be ingested as part of diet.