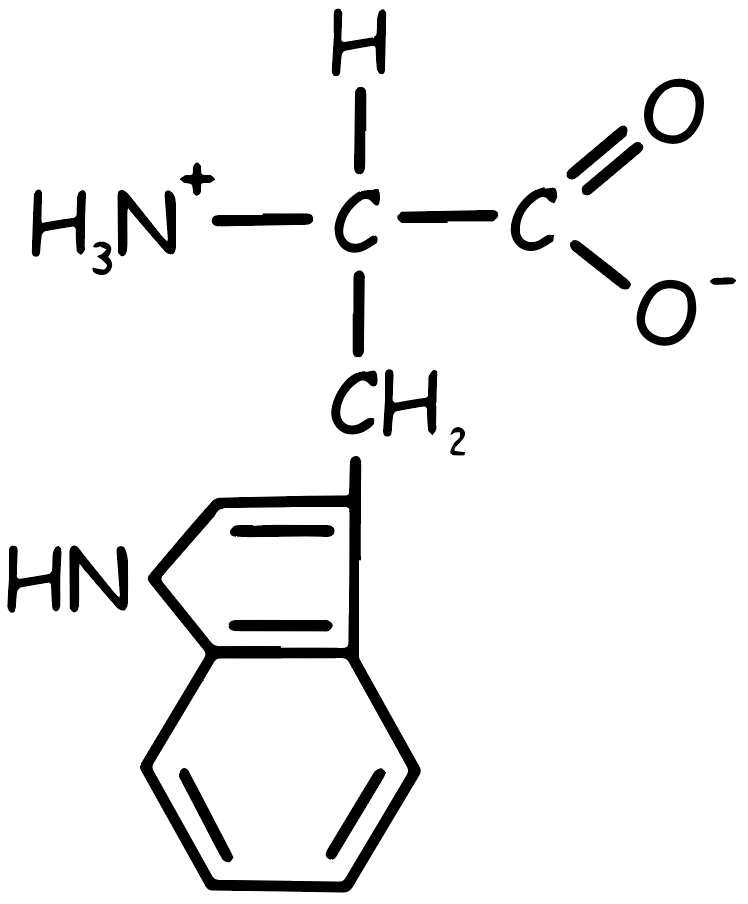
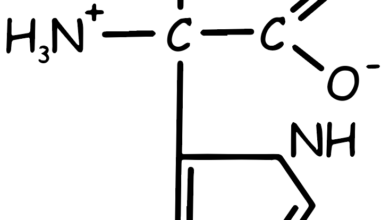Tryptophan Amino Acid
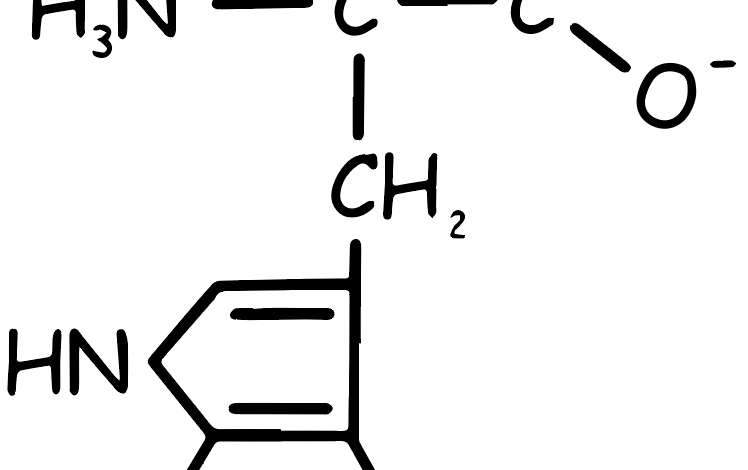
This essential amino acid, characterized by two rings in its side chain, is non-polar and has a large side chain that prevents it from fitting into an alpha helix. It cannot be synthesized in the body, making it a necessary dietary component. Tryptophan is found in foods like nuts, cheese, red meat, chicken, fish, oats, beans, lentils, and eggs.
Tryptophan’s catabolic pathway leads to the formation of Alanine, which can then transform into pyruvate through transamination. This makes Tryptophan a glucogenic amino acid. Additionally, Tryptophan can convert into acetoacetyl CoA, a ketone body, thereby classifying it as ketogenic as well.
Serotonin Synthesis
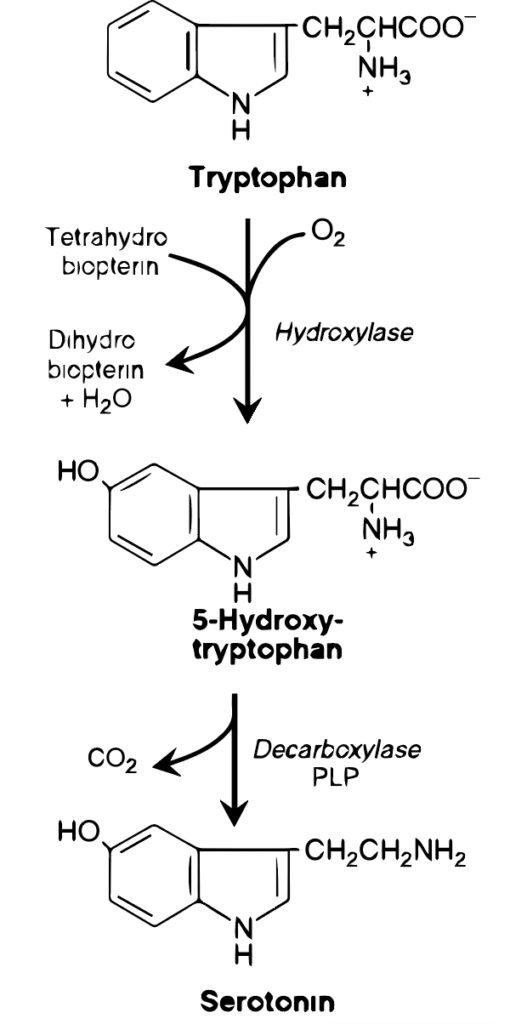
Serotonin synthesis from tryptophan is a biochemical process that occurs in the body (see Figure 2). After digestion, tryptophan enters the bloodstream and crosses the blood-brain barrier to enter the brain. In the brain, tryptophan is converted into 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase. This is the rate-limiting step in serotonin synthesis, meaning it’s the step that most controls the speed of the process. 5-HTP is then converted into serotonin (5-hydroxytryptamine, or 5-HT) by the enzyme aromatic L-amino acid decarboxylase. Factors like nutrient availability, levels of stress, and the presence of certain cofactors (like vitamin B6, which is necessary for the function of aromatic L-amino acid decarboxylase) can influence the efficiency of this process.
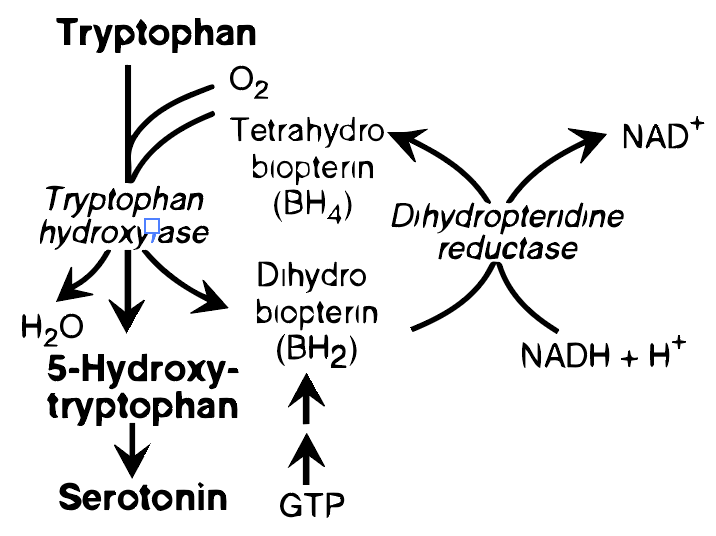
Serotonin, often known as 5-hydroxytryptamine (5HT), is produced and stored in various parts of the body (see Figure 3). The majority of serotonin resides in the intestinal mucosa cells. Lesser quantities are found in the central nervous system, where it acts as a neurotransmitter, and in blood platelets. The synthesis of serotonin begins with tryptophan, which undergoes a BH4-dependent hydroxylation process similar to the one facilitated by phenylalanine hydroxylase. This leads to the formation of 5-hydroxy tryptophan, which is then decarboxylated to create serotonin. Serotonin is also broken down by MAO. It plays several physiological roles, including influencing pain perception, regulating sleep, appetite, body temperature, blood pressure, cognitive functions, and mood, often inducing a sense of well-being. [Note: In the pineal gland, serotonin undergoes acetylation and methylation to transform into melatonin.]
Niacin (Vitamin B3) Synthesis
Niacin, also known as Vitamin B3, can be synthesized in the human body from the amino acid tryptophan. The major pathway for tryptophan catabolism is the kynurenine pathway. In this pathway, tryptophan is converted into kynurenine by the enzyme tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO). Kynurenine undergoes several more transformations through a series of enzymatic steps. It is eventually converted into quinolinic acid, which is then used to synthesize nicotinic acid (niacin). The niacin produced can be utilized by the body in various forms, including nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), which are essential coenzymes in cellular metabolic reactions.
It’s important to note that the conversion of tryptophan to niacin is not highly efficient; it is estimated that it takes about 60 mg of tryptophan to produce 1 mg of niacin. Additionally, several factors, including the presence of other nutrients and overall health, can influence this conversion process. This is why niacin is also an important component of the diet, found in foods like meats, fish, nuts, and grains.
Hartnup Disease
An autosomal recessive, rare disorder, first identified in a particular family, involves a defect in the SLC6A19 gene on chromosome 5, which encodes a neutral amino acid transporter responsible for transporting tryptophan. This defect leads to an inability to absorb tryptophan from the intestine and reabsorb it from the kidneys, resulting in aminoaciduria, characterized by the presence of tryptophan in urine without a corresponding increase in plasma levels. Despite adequate dietary intake of tryptophan and niacin, patients exhibit Pellagra-like symptoms, including neurological and dermatologic issues, and are part of Garrod’s tetrad. Typically manifesting around 30-40 years of age with lower backache but without mental retardation, patients’ fresh urine appears normal in color, but Benedict’s test is positive due to the presence of reducing substances like Homogentisic acid, and FeCl3 test is also positive, often leading to bluish urine stained diapers. Treatment includes niacin supplementation and a high-protein diet.
Carcinoid Syndrome
Carcinoid syndrome is a condition that affects 5% of individuals with carcinoid tumors, which are known for producing serotonin. In these cases, a significant amount of Tryptophan is utilized for serotonin production, leading to a decrease in Tryptophan available for niacin synthesis. This results in symptoms similar to pellagra. The clinical features of carcinoid syndrome include excessive sweating, skin flushing, disturbances in gastrointestinal motility, abdominal cramps, and diarrhea. Additionally, there is an elevated level of 5-Hydroxy Indole Acetic Acid (5-HIAA) in the urine.